You are currently viewing the James Walker Australia & New Zealand website. Click here to view the Global website.
You are currently viewing the James Walker Australia & New Zealand website. Click here to view the Global website.
What is the impact of choosing sealing components created to deliver high performance?
Wednesday, 4 January 2023Regulations and guidelines in biopharmaceutical processing greatly assist drug corporations when developing processes and procedures for the creation and manufacture of quality, safe and effective therapeutic products.
Within FDA and USP guidelines, compliance is a minimum standard, however there is a limited defined ceiling to a component’s quality and performance. This article seeks to explore the benefits to industry in choosing sealing components created to deliver higher performance levels, and asks the question - what is the impact of such an approach?
EPDM materials
EPDM (ethylene propylene diene monomer) is a base polymer commonly used in the manufacture of seals in biopharmaceutical systems. To get the desired properties in terms of performance and regulatory requirements, other materials are added to this base polymer. These can include plasticisers, carbon black and inorganic fillers. The exact detail of these additives (and the quantities used) has a strong influence on the material performance, and cleanliness.
One method to investigate the various constituents of the different material combinations (so-called formulations) is thermogravimetric analysis (TGA) in which a small sample is heated to high temperature, under a controlled atmosphere, resulting in a stepwise degradation/volatisation of the individual components.
The chart below shows the TGA traces of two EPDM formulations both employed in biopharmaceutical applications. Consequently, both materials satisfy the appropriate regulatory requirements (USP <87>, USP <88>, FDA 21 CFR 177.2600, etc.). However, it is very clear that the two materials vary greatly in their chemical make-up, and consequently their mechanical performance. 
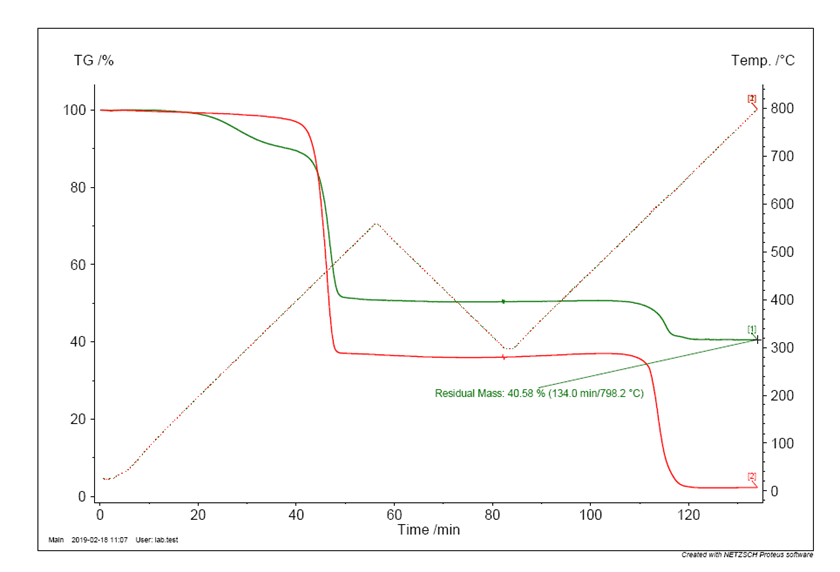
Firstly consider the TGA trace for Elast-O-Pure EP75B (the red line). Heating under nitrogen shows an initial weight loss of ca. 62% w/w corresponding to the EPDM polymer degradation, followed by a second weight loss, after switching to an air atmosphere, of ca. 34% w/w due to the carbon black content. This leaves ca. 4% w/w of inorganic material residue.
The competitor material (as indicated by the green line) shows a much more complex TGA profile, with a three stage degradation, indicating a more complex mixture. The first stage shows a weight loss of ca. 10% w/w, at relatively low temperatures. This is normally associated with plasticisers being present, which are included to aid processing of the material. Unfortunately, plasticisers are not bound into the polymer matrix and are free to migrate out of the material, giving a likely increase in extractable content. The second degradation of ca. 40% w/w is the base EPDM breakdown, with the final degradation of ca. 10% w/w associated with the carbon black content, which is low for an EPDM formulation. This leaves an unusually high inorganic content of ca. 40% w/w.
These two facts, low carbon black and high inorganic content, together raise doubts over the robustness of this material. Carbon black is added to rubber to enhance the properties (it reinforces the matrix), however addition levels of ca. 10% w/w will not give this reinforcement. Conversely, inorganic fillers do not generally enhance mechanical properties, but tend to reduce them, so low carbon black and high inorganic additives will seriously compromise the mechanical properties. Such high levels of inorganic additives are normally employed to reduce the bulk cost of the material, at the expense of material performance.
Conclusion
It is clear that although both of the tested materials meet the regulatory requirements, their composition, and mechanical properties will be totally different. The competitive material to Elast-O-Pure EP75B showed the following:
1. The presence of plasticisers, which by their very nature will leach out, potentially contaminating the process, and potentially leading to costly batch loss.
2. Low levels of carbon black, and high inorganic additions, both of which will reduce the mechanical properties which may result in;
Naturally Elast-O-Pure does not contain plasticisers and minimal inorganic filler levels as shown above. Consequently Elast-O-Pure is a higher performance material, offering benefits such as eliminating the need to re-torque with minimal intrusion leading to improved cleanability and greater in-service life span, in some cases up to 3 years.
Choosing a sealing component created to deliver higher performance is therefore an important consideration in biopharmaceutical processing.

