You are currently viewing the James Walker Australia & New Zealand website. Click here to view the Global website.
You are currently viewing the James Walker Australia & New Zealand website. Click here to view the Global website.

Selecting a sealing material with low compression set can have a significant positive impact on biopharma plant productivity and costs.
Thursday, 11 January 2024In materials science, compression set is a measure of the residual deformation in an elastomer after a compressive force is removed. Compression set results for a material are expressed as a percentage; a lower compression set is considered better as it indicates that the material will retain more of its original shape, and properties, after being compressed over time. Conversely, a higher compression set implies that the material can suffer a loss of mechanical properties resulting in permanent deformation.
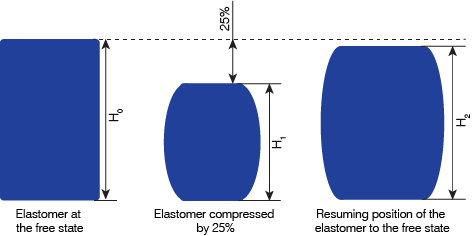
ASTM D395 has two methods of determining compression set, A and B. James Walker employs method B, in which compression set is determined by subjecting a specimen to a constant compression (to 75% of its original height) for a set time and temperature. The percentage of the specimen's original thickness that it retains after being released from the compression and then allowed to relax is then measured.
Compression set = {(H0 - H2) / (H0 - H1)} x 100
A lower compression set is preferred in applications where long-term resilience and durability are critical, such as in seals and gaskets; any resulting permanent set that a gasket may take, caused by a high compression set material over time, may cause a leak.
Elast-O-Pure EP75B has been specifically engineered for the biopharma industry, with a uniquely low compression set value, resulting in seals that are highly durable and long-lasting. Additionally, after an initial fitting, the seals offer a "fit-and-forget" advantage, with no re-torqueing required, even after 500 SIP cycles.
Measurement of the compression set of other materials used in biopharma sealing applications gave the following results:
| Material |
Compression set 24hrs / 100°C |
Compression set 24hrs / 125°C |
|---|---|---|
| Elast-O-Pure EP75B | 4% | 6% |
| Material A | 19% | 31% |
| Material B | 19% | 27% |
| Material C | 30% | 39% |
| Material D | 20% | 30% |
*As determined by ASTM D395 Method B
Experience in the field has proven the value of choosing Elast-O-Pure EP75B, however, what are the implications of the very high compression set results of the other materials in a biopharma application?
As already stated, high compression set materials are prone to movement under load as well as taking a permanent set, and these two factors have a serious effect on the performance of tri-clamp seals universally employed throughout the industry.
The most obvious effect of high compression set on these seals is that the seal will be permanently reduced in thickness. This in turn will reduce the sealing force on the seal, which, if the pressure in the system is high enough, can result in a leak. To prevent this, re-torqueing is required to maintain the sealing force, however, this causes a further reduction in thickness of the seal and an additional need to re-torque, and so on. On a typical size plant, with 10,000 gaskets, the cost of this re-torqueing operation is significant.
This reduction in seal thickness displaces some of the elastomeric material, which due to the seal design has no option but to move, and intrude, into the process flow. This can result in significantly reduced flow, as well as a build-up of process materials behind the intruded section making cleaning difficult, thereby raising the possibility of contamination between batches. Additionally, any intruded material is more prone to breaking off into the process flow, particularly during SIP cycles, and blocking filters further downstream.
This movement also limits the useful life of the seal, resulting in more change-outs being required, along with the impact of an associated increase in downtime and resultant reduced output.
In summary, use of high compression set materials for seals in biopharma operations can result in:
Conclusion
Ultimately, the ability to manufacture more product from existing resources, whilst reducing both maintenance and spares costs, is the key objective for most biopharma manufacturing and site leads. A low compression set on tri-clamp gaskets is a critical factor in being able to achieve this.
James Walker’s Elast-O-Pure tri-clamp gaskets and O-rings have been trusted in the manufacture of biologic product for over 15 years. During this time, a number of established global customers have highlighted the benefits of our partnership and the value that they have realised through shared knowledge and expertise.
These include:
Evidence that making the decision to opt for a sealing material with low compression set value can have a proven, significant impact on productivity and costs.


Want to discuss your project, engineering or materials challenge expert to expert? Simply provide us with your contact details and a little information about the application you are working on, and one of our experts will contact you as soon as possible.