You are currently viewing the James Walker Global website.
You are currently viewing the James Walker Global website.

Improve quality, efficiency and cost control by improving sealing performance.
Tuesday, May 10, 2022Contract manufacturing in biopharma results in several different products being manufactured on the same process line. This has obvious implications for cross contamination, therefore the cleaning regime needs to be very thorough. Taking a strict risk averse approach, this often involves the changeout of each rubber seal to minimise to potential of contamination from campaign to campaign. Not only is this expensive in terms of replacement seals, but also in maintenance time and reduced capacity due to the excess downtime required to carry out this operation.
This has been brought about by the use of commodity, low cost, sealing materials, which were not specifically engineered for biopharma applications, and may only be suitable for one campaign because they cannot withstand the rigors of the CIP process. Given that typically the key challenges in contract manufacturing are quality, efficiency and cost control, is it worth considering an approach that can address all of these areas?
Our Elast-O-Pure EP75 Black has, uniquely, been specifically designed for biopharma applications and as such is easily able to cope with the cleaning regimes employed, being resistant to the cleaning chemicals employed and WFI steam.
For example, Elast-O-Pure EP75 Black was exposed to 500 'typical' CIP cycles, as shown below:
This gives a total exposure of 333 hours in hot water, 375 hours in hot caustic and 500 hours in steam. The important point to note about this test cycle is the steam temperature of 135°C, which is the top end of SIP temperatures typically used.
The result of such testing showed the Elast-O-Pure EP75 Black gasket to be undamaged.
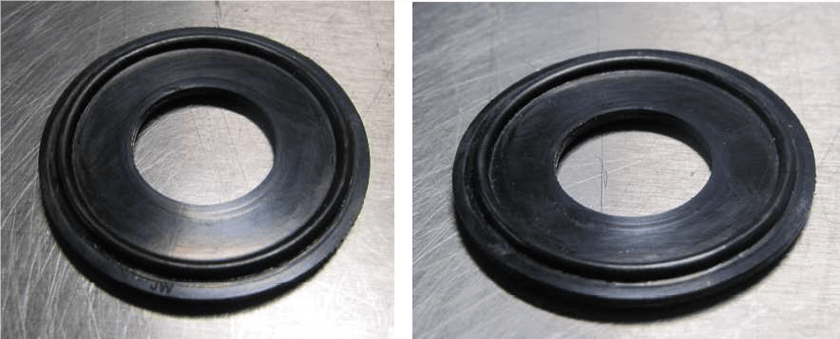
These pictures show the virgin seal (left) and the seal after 500 test cycles (right), demonstrating no visible damage to the tested seal.
More importantly, no retorque was carried out over the test duration, not even once, and no leaks were detected. This is a significant result from a maintenance and downtime standpoint as many biopharma companies need to retorque after the initial steam cycle, or experience leaks when using commodity gaskets. With a typical plant having, say, 10,000 gaskets, the cost and time, to retorque is significant, not to mention the increased downtime, and hence reduced capacity and return on capital investment.
The benefits of using specifically designed high performance materials and their role in avoiding campaign driven change outs in a multiproduct environment are significant. Data supplied by end users indicated the following advantages;
Finally, the hygienic clamp seals, made from Elast-O-Pure EP75 Black, have been specifically designed to minimise intrusion into the pipe bore, even after steam exposure, thereby ensuring easy cleaning and eliminating any cross contamination when a suitable cleaning regime is employed.
In conclusion, reviewing the quality and methodology of your hygienic clamp seals and O-rings in the production of large molecule drugs has the potential to maximise plant efficiency and minimise maintenance time and cost, helping you and your customers to more easily meet market demand.

Want to discuss your project, engineering or materials challenge expert to expert? Simply provide us with your contact details and a little information about the application you are working on, and one of our experts will contact you as soon as possible.